Background: Acquired somatic mutations are crucial for the development of the majority of cancers. In hematological malignancies, some molecular mutations are very specific for certain entities (e.g. BRAF in HCL, MYD88 in LPL), while others were detected in a variety of malignancies (e.g. mutations in TP53, TET2, DNMT3A, RUNX1). Moreover, mutations in genes related to CHIP (clonal haematopoiesis of indeterminate potential; ASXL1, TET2,DNMT3A) were detected in an age-related manner.
Aim: (1) Analysis/comparison of mutation frequencies of 122 selected genes in 3096 cases with 28 different hematological malignancies for identification of "mutation-driven" entities. (2) Correlation of CHIP-related mutations with mutational landscapes.
Methods: Whole-genome sequencing (WGS) was performed for all 3096 patients. For this, 151bp paired-end reads were generated on NovaSeq 6000 and HiSeqX machines (Illumina, San Diego, CA). The Illumina tumor/unmatched normal workflow was used for variant calling. All reported p-values are two-sided and were considered significant at p<0.05.
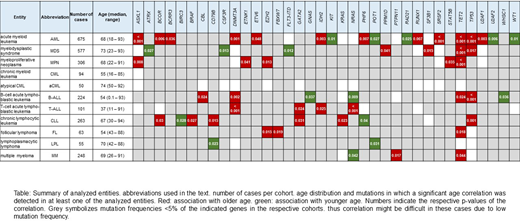
Results: Entities with the highest numbers of mutations (median n=4) and thus potentially with the largest impact of mutations on pathogenesis comprised aCML (range: 1-7), CMML (1-6), MDS/MPN-U (2-5) and s-AML (2-8), whereas the lowest numbers (median n=0) were observed for CML (0-3), MGUS (0-2), MLN_eo (0-3), NK cell neoplasm (0-3) and PPBL (0-2). In the total cohort of 3096 cases, the most frequently mutated genes were TET2 (14%), ASXL1 (13%), TP53 (10%), SF3B1 (9%), DNMT3A (9%) and SRSF2 (9%). Entities with high frequencies of specific mutations (> 50%) comprised: aCML (ASXL1, 86%), BPDCN (TET2, 67%), BL (TP53, 60%), CMML (TET2, 67%; ASXL1, 58%), FL (KMT2D, 87% and CREBBP, 73%), HCL (BRAF, 100%), LPL (MYD88, 98%; CXCR4, 51%), MDS/MPN-U (ASXL1, 60%), MPN (JAK2, 68%), B-NHL (TP53, 50%) and T-NHL (STAT3, 52%). Mutations enriched in distinct entities included SETBP1 (26% in MDS/MPN overlaps), CSF3R (30% in MDS/MPN-U), STAT3 (only in T-NHL and NK cell neoplasm, 52% and 23%), NOTCH1 and PHF6 (T-ALL, 38% and 30%) and MYC and ID3 (almost exclusively in BL, 30% each). Genes predominantly mutated in myeloid neoplasms comprised e.g. SF3B1 (with the exception of CLL), JAK2, NPM1, RUNX1, IDH2, CEBPA, STAG2, NF1 and GATA2. By contrast, mutations in KMT2D, MYD88, ARID1A, ATM, CXCR4, BIRC3 and CD79B were detected almost exclusively in lymphoid malignancies. A broad distribution across entities was observed for mutations in TET2, ASXL1,DNMT3A, TP53, BCOR and ETV6. Thus, the first three, i.e. CHIP-related genes were also mutated with a high frequency in lymphoid neoplasms. In line with this, gene mutations found in the largest number of entities comprise DNMT3A (n=23 entities), TET2 (n=21), ASXL1, TP53, NRAS (n=19, respectively), KRAS and BCOR (n=17, respectively). Further, we compared the mutational patterns of cases with at least one CHIP-associated mutation (n=920 cases in the total cohort, "CHIP+") with cases without such mutations (n=2176, "CHIP-") to decipher CHIP-correlated mutation patterns. Significant differences with respect to accompanying mutations were mainly detected for myeloid neoplasms (MDS, mutations in n=12 genes significantly different in CHIP+ vs. CHIP- without CHIP genes themselves; AML, n=7; MPN, n=4; aCML, n=2; CMML, n=1) but also for MPAL (n=3), T-ALL (n=2), B-ALL, FL and LPL (n=1, respectively). Mutations in TP53 were found significantly enriched in CHIP- cases in 4 different entities, moreover mutations in KRAS, WT1 and SF3B1 were more abundant in CHIP- cases (in CMML, AML and aCML, respectively). By contrast, CHIP+ cases were characterized by high frequencies of mutations in RUNX1 (in n=4 entities), SRSF2, IDH2, NRAS (n=3) and EZH2 (n=2).
Conclusions: (1) Certain mutations showed a broad distribution within or even across the myeloid/lymphoid lineage, including CHIP-related mutations frequently detected also in lymphoid malignancies. (2) The median numbers of mutations were low in entities that are defined by chromosomal fusions (CML, MLN_eo) or in entities that are regarded as "pre-malignant" (MGUS, PPBL), while especially MDS/MPN overlap cases seem to be mutation-driven (high number of mutations). (3) We deciphered different mutation patterns in CHIP+ (RUNX1, SRSF2, IDH2, NRAS, EZH2) and CHIP- (TP53, KRAS, WT1, SF3B1) cases across all entities, suggesting differences in pathophysiology.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal